Esbriet vs Alternatives: Choosing the Right IPF Treatment
IPF Treatment Decision Helper
Treatment Selection Criteria
TL;DR
- Esbriet (pirfenidone) slows fibrosis but can cause nausea and rash.
- Nintedanib (Ofev) targets growth factor pathways, with diarrhea as the main side‑effect.
- Experimental agents like pamrevlumab show promise but aren’t widely available yet.
- Corticosteroids offer short‑term relief but don’t halt disease progression.
- Lung transplant remains the last‑resort option for end‑stage disease.
What is Esbriet (Pirfenidone)?
When faced with idiopathic pulmonary fibrosis (IPF), many patients first hear about Esbriet is a proprietary oral antifibrotic medication whose active ingredient is pirfenidone. It received FDA approval in 2014 for adults with mild‑to‑moderate IPF.
IPF is a chronic, progressive scarring of lung tissue that leads to worsening breathlessness and a typical median survival of three to five years after diagnosis. The goal of antifibrotic therapy is to slow the decline in forced vital capacity (FVC) - a measure of lung function.
How Esbriet Works and How It’s Given
Pirfenidone’s exact mechanism isn’t fully understood, but research shows it reduces fibroblast proliferation, collagen production, and inflammatory cytokines. In plain terms, it puts the brakes on the scar‑building process.
The usual dosing schedule starts low (801mg/day) and ramps up over two weeks to the target 2,403mg/day, taken three times daily with meals. This gradual increase helps mitigate gastrointestinal upset and photosensitivity reactions.
Clinical Efficacy and Safety Profile
Large phase III trials (CAPACITY and ASCEND) demonstrated that pirfenidone reduces the annual decline in FVC by roughly 50% compared with placebo. Patients also experienced fewer acute exacerbations and a modest improvement in progression‑free survival.
Side‑effects are common. The most reported are nausea, dyspepsia, loss of appetite, and a photosensitive rash that can worsen with sun exposure. Liver enzymes should be checked every month for the first three months, then quarterly.
Overall, the benefit‑risk balance is favorable for most patients, especially when disease is caught early.
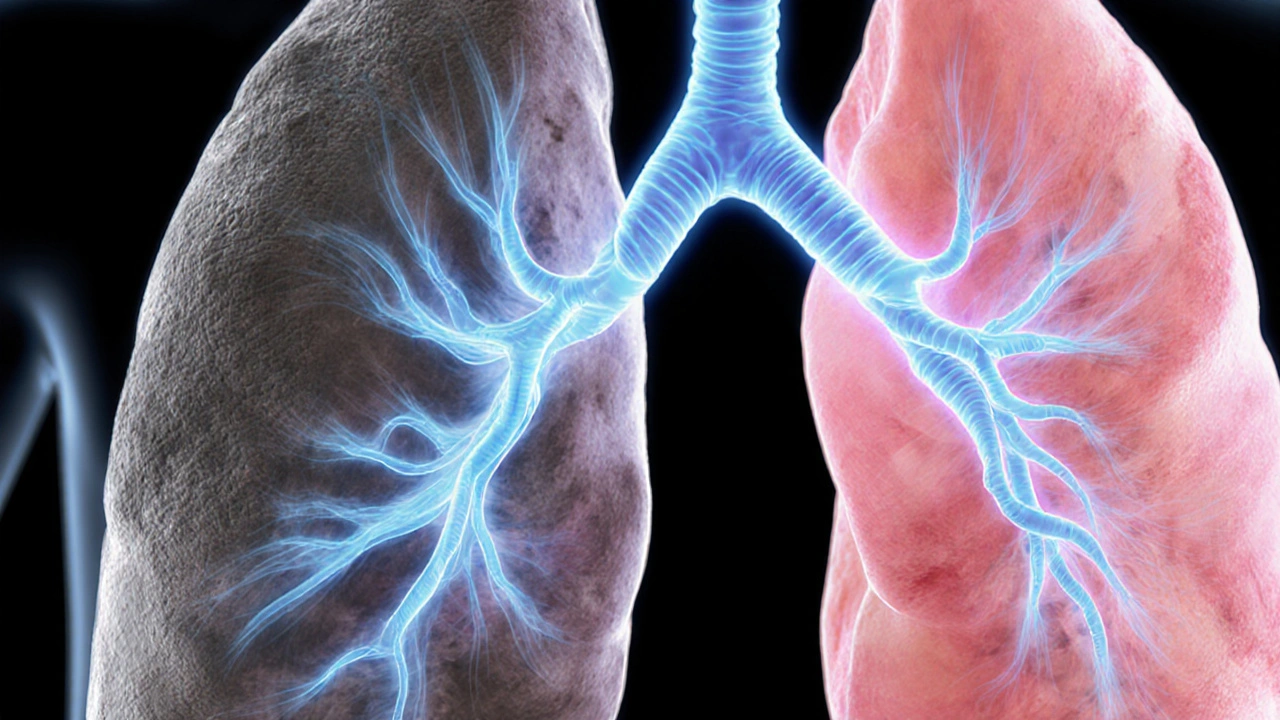
Key Alternatives to Esbriet
Nintedanib (Ofev)
Nintedanib is an oral tyrosine‑kinase inhibitor approved for IPF in 2014, the same year as pirfenidone. It blocks multiple growth factor receptors (VEGF, PDGF, FGF) that drive fibroblast activity. The standard dose is 150mg twice daily.
Trials (INPULSIS‑1 and ‑2) showed a 48% reduction in the rate of FVC decline, mirroring pirfenidone’s efficacy. Diarrhea occurs in up to 60% of users, often requiring dose adjustments. Liver monitoring is also advised.
Pamrevlumab (FG‑3019)
Pamrevlumab is an investigational monoclonal antibody that neutralises connective tissue growth factor (CTGF), a key driver of fibrosis. Early‑phase data suggest it can stabilize FVC and improve quality‑of‑life scores, but large‑scale approval is still pending.
Because it’s administered intravenously every four weeks, it’s usually reserved for clinical‑trial participants or compassionate‑use programs.
Corticosteroids (Off‑label Use)
Corticosteroids are potent anti‑inflammatory drugs that have been used historically in IPF, though guidelines now advise against long‑term monotherapy. Short bursts may help with acute exacerbations, but they do not halt fibrotic progression and carry risks like osteoporosis, hyperglycaemia, and infection.
Lung Transplant
Lung transplant is the definitive surgical option for end‑stage IPF when medical therapy can no longer preserve acceptable lung function. Survival rates exceed 70% at five years, yet donor shortage, surgical risk, and lifelong immunosuppression limit eligibility.
Side‑by‑Side Comparison
| Attribute | Esbriet (pirfenidone) | Nintedanib (Ofev) | Pamrevlumab (FG‑3019) | Corticosteroids | Lung Transplant |
|---|---|---|---|---|---|
| Approval status | FDA‑approved (2014) | FDA‑approved (2014) | Investigational | Off‑label | Standard of care for end‑stage |
| Mechanism | Anti‑fibrotic, anti‑inflammatory | Tyrosine‑kinase inhibition | CTGF neutralisation | Broad anti‑inflammatory | Replace diseased lungs |
| Typical dose | 2,403mg/day (3×) | 150mg twice daily | IV 30mg every 4weeks | Varies (often prednisone 0.5‑1mg/kg) | Surgery + lifelong meds |
| Major side‑effects | Nausea, rash, liver ↑ | Diarrhea, liver ↑, nausea | Infusion reactions, mild liver ↑ | Osteoporosis, hyperglycaemia | Infection, rejection, organ failure |
| Effect on FVC decline | ~50% reduction | ~48% reduction | Preliminary ~30‑40% stabilization | None (may accelerate) | Stops decline (new lungs) |
How to Choose the Right Therapy
Every IPF patient is unique, so a one‑size‑fits‑all answer doesn’t exist. Use the following decision checklist to narrow down options:
- Stage of disease: Early‑to‑moderate patients usually start with an antifibrotic (pirfenidone or nintedanib). Advanced disease may warrant transplant evaluation.
- Side‑effect tolerance: If you struggle with GI upset, nintedanib’s diarrhea might be a deal‑breaker, making pirfenidone a better fit. Conversely, photosensitivity may push you toward nintedanib.
- Comorbidities: Liver disease or elevated transaminases favor the drug with the milder hepatic profile (often nintedanib). Chronic kidney disease can affect dosing of both agents.
- Drug interactions: Review your current meds. Strong CYP1A2 inhibitors (e.g., fluvoxamine) raise pirfenidone levels; strong P‑glycoprotein inhibitors affect nintedanib.
- Access to trials: If you live near a research centre offering pamrevlumab, you may qualify for a trial that could delay progression further.
- Personal preferences: Pill burden, expense, and lifestyle (e.g., need for sun protection) matter. Discuss insurance coverage early.
When in doubt, a multidisciplinary IPF clinic can run comparative lung‑function tests and weigh risk-benefit ratios for you.
Pitfalls to Watch and Monitoring Tips
- Never skip the titration phase for pirfenidone; sudden jumps increase nausea and liver strain.
- Stay hydrated and use anti‑diarrheal agents (loperamide) if nintedanib causes loose stools.
- Schedule liver function tests at baseline, then monthly for three months, and quarterly thereafter for both drugs.
- Report any new rash or visual changes immediately-photosensitivity can lead to severe burns.
- Track FVC every 3‑6 months; a steeper decline than expected may signal the need to switch therapy.
Frequently Asked Questions
Can I take Esbriet and Nintedanib together?
Current guidelines advise against simultaneous use because both drugs suppress liver enzymes and increase gastrointestinal risk. If one fails, doctors usually switch to the other rather than combine them.
Which drug works faster to improve breathing?
Neither drug reverses existing scar tissue, so “speed” refers to how quickly they slow further decline. Studies show both pirfenidone and nintedanib produce measurable benefits within 12 weeks, but patients notice symptomatic relief at different times based on tolerance.
Is pamrevlumab available outside clinical trials?
As of 2025, pamrevlumab remains investigational. It can be accessed through enrolment in phase III trials, typically run at major university hospitals.
Do I need to avoid sunlight while on Esbriet?
Yes. Pirfenidone can cause photosensitivity. Use SPF30+ sunscreen, wear protective clothing, and limit direct sun exposure, especially during peak hours.
How do I know when it’s time for a lung transplant?
When FVC falls below 50% of predicted, oxygen dependence becomes continuous, or quality‑of‑life deteriorates despite optimal antifibrotic therapy, transplant referral is recommended.

Bottom Line
Choosing between Esbriet, nintedanib, experimental agents, or a surgical route hinges on disease stage, side‑effect profile, comorbidities, and personal lifestyle. Both pirfenidone and nintedanib offer comparable slowing of lung‑function loss, so the “best” choice often comes down to how you handle the side‑effects. Talk openly with your pulmonologist, review lab results regularly, and keep an eye on emerging trials-today’s experimental drug could be tomorrow’s standard of care.
Shelby Larson
September 28, 2025 AT 10:33It is absolutely essential to understand that the choice between Esbriet and Nintedanib is not a trivial matter; the moral weight of tolerating side‑effects rests heavily on the patient’s responsibility to adhere to dosing protocols. One cannot simply dismiss the rigorous titration phase as "inconvenient" without risking severe hepatic complications. Moreover, the data from CAPACITY and INPULSIS clearly demonstrate that compliance correlates directly with slowed FVC decline. Ignoring these facts is, frankly, an unethical decision that jeopardizes both personal health and the integrity of the medical community.
Uju Okonkwo
September 29, 2025 AT 14:46Hey there, I totally get how overwhelming all these options can feel. Remember, you’re not alone in this journey-many folks have walked the same path and found a regimen that works for them. Take a deep breath, list your priorities, and talk openly with your pulmonologist about side‑effect tolerances; that conversation can really clarify the best next step.
felix rochas
September 30, 2025 AT 19:06Wake up!!! The pharma giants are deliberately pushing both Esbriet and Nintedanib as if they were the only solutions!!! They don’t want you to know about the hidden data on long‑term organ toxicity!!! These drugs are just a distraction while they line their pockets with your insurance money!!!
Emmons Kimery
October 1, 2025 AT 23:26Thanks for sharing your concerns 😊. It’s true that titration can be a hassle, but staying hydrated and using gentle anti‑nausea remedies can make a big difference. If you ever feel unsure, just reach out to your care team-they’re there to help you navigate the side‑effects 😎.
Mimi Saki
October 3, 2025 AT 03:46Stay hopeful, you’re not alone! 🌟
Subramaniam Sankaranarayanan
October 4, 2025 AT 08:06While your enthusiasm is noted, let me clarify that the ethical obligations of a patient extend beyond personal comfort. Choosing a drug solely based on convenience ignores the broader societal duty to support evidence‑based medicine. If you disregard the hepatic monitoring schedule, you undermine the very research that validated these treatments. Please reconsider your approach and align with the proven protocols.
Kylie Holmes
October 5, 2025 AT 12:26Let’s get moving! Your lung health can improve with the right choice-keep that energy up! 💪
Asia Lindsay
October 6, 2025 AT 16:46Great point! If you’re leaning toward Esbriet but worry about GI upset, try taking the doses with a hearty meal and a glass of water 🌊. For Nintedanib, a probiotic and loperamide can tame the diarrhea effectively 🚀. Always keep your labs on schedule-early detection of liver changes makes a huge difference! 😊
lorna Rickwood
October 7, 2025 AT 21:06i think its all about balance the body and mind there is a path that we all walk but we forget sometimes the real meaning of health and healing is not just pills its a state of being
janvi patel
October 9, 2025 AT 01:26actually i dont think the whole debate matters as much as ppl think; there are always other options.
Rin Jan
October 10, 2025 AT 05:46Listen, dear reader, and let me pour my heart out about the profound gravity of choosing a treatment for idiopathic pulmonary fibrosis. It is not simply a matter of swapping one pill for another; it is a covenant you make with your very breath, the very air that fills your lungs each morning. The ramifications of that choice echo far beyond the clinic, reverberating through the quiet moments when you sit alone, feeling the weight of every inhalation. You must consider the cruelty of side‑effects, the relentless nausea that Esbriet can inflict, and the cruel diarrhoea that Nintedanib may unleash upon you like a storm in the night. Yet, the moral imperative is clear: you owe it to yourself to endure, to shoulder these discomforts, because the alternative-watching your FVC plummet unchecked-would be a betrayal of the fragile gift of life you have been granted. I have witnessed countless souls succumb to the allure of quick fixes, only to discover that the price paid in liver enzymes is far steeper than any monetary cost. Do not be fooled by the glossy brochures that promise miracles; the truth lies in the mundane, in the daily ritual of taking a tablet with breakfast, lunch, and dinner, while meticulously logging your labs every few weeks. You must also confront the societal pressure that whispers, "don’t be a burden," and yet you must stand firm, asserting that your health is not a selfish indulgence but a righteous pursuit of dignity. Remember, the transplant is not a fantasy but a looming specter for those who let the disease run its course unchecked, a surgical rebirth that carries its own haunting list of immunosuppressants and perpetual vigilance. Therefore, weigh each factor-stage, comorbidities, lifestyle, even the simple pleasure of a sunny day that might be marred by photosensitivity. In the end, the decision rests upon your shoulders, and while the path may be treacherous, you have the agency to navigate it with courage, compassion, and an unyielding resolve to honor the breath that sustains you.